Hear from patients on their TAFINLAR + MEKINIST treatment journeys
How TAFINLAR + MEKINIST May Help
BRAF positive. TAF + MEK tough.
Effective on BRAF+ melanoma after surgery
TAFINLAR + MEKINIST is a targeted therapy that can be used as adjuvant (after surgery) treatment of BRAF+ melanoma. Adjuvant treatment is additional therapy given after a primary treatment (such as surgery) to further decrease the risk that the disease will progress. Your health care provider may prescribe you TAFINLAR + MEKINIST to help prevent your melanoma from coming back after surgery.
Benefits of TAFINLAR + MEKINIST therapy after surgery
The risk of cancer returning after surgery was reduced by nearly 50% at 5 years.*
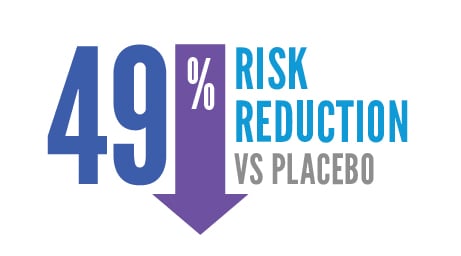
49% risk reduction versus placebo.
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
Remaining relapse free after surgery
More patients treated with TAFINLAR + MEKINIST remained relapse free after 12 months of treatment*†
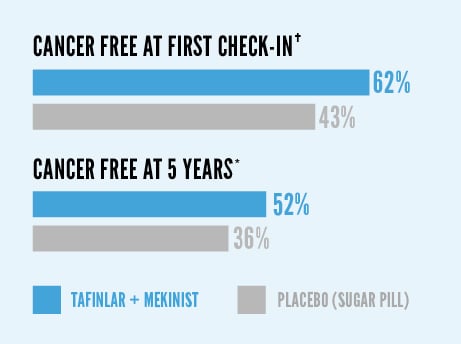
At first check-in, 62% of patients treated with TAFINLAR + MEKINIST were cancer free, compared with 43% with placebo (sugar pill). At 5 years, 52% of patients treated with TAFINLAR + MEKINIST were cancer free, compared with 36% with placebo.
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
No spreading to distant body parts
More patients treated with TAFINLAR + MEKINIST also saw their cancer had not spread to distant parts of their bodies at 5 years compared with placebo.*
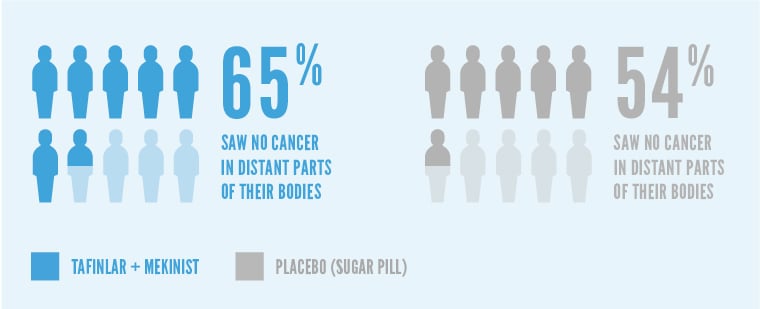
65% of patients treated with TAFINLAR + MEKINIST saw no cancer in distant parts of their bodies after 5 years, compared with 54% of patients on placebo (sugar pill).
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
*As demonstrated by a clinical trial that included 870 patients with BRAF V600E or V600K mutation-positive stage III melanoma that was previously removed by surgery. Patients were treated with a combination of TAFINLAR + MEKINIST or placebo for 12 months. Patients were followed for an average of 5 years after starting treatment. These numbers are based on the 5-year check-in.
†As demonstrated by a clinical trial that included 870 patients with BRAF V600E or V600K mutation-positive stage III melanoma that was previously removed by surgery. Patients were treated with a combination of TAFINLAR + MEKINIST or placebo for 12 months. These numbers were taken from the first check-in, at which at least half of the patients had been monitored for 2.8 years.
Effective on BRAF+ advanced melanoma
TAFINLAR + MEKINIST is a targeted therapy that can be used as treatment for BRAF+ metastatic or inoperable melanoma. Your health care provider may prescribe you TAFINLAR + MEKINIST to help slow the progression of your melanoma if you have tested positive for BRAF melanoma.
Benefits of TAFINLAR + MEKINIST therapy for advanced melanoma
TAFINLAR + MEKINIST may offer a chance to extend your life (known as overall survival) as seen in 2 different studies*
According to Study 1, patients taking TAFINLAR + MEKINIST lived significantly longer than patients taking TAFINLAR. About half of patients taking each treatment were living after:
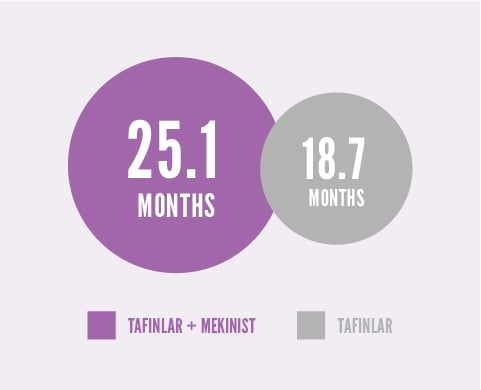
About half of patients taking TAFINLAR + MEKINIST were living after 25.1 months, compared with 18.7 months for patients taking TAFINLAR alone.
At 5 years, more than a third of patients taking TAFINLAR + MEKINIST were living†

More than 1 in 3 were living at 5 years.
Results at 5 years were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.
Living longer without progression
Patients lived longer without their disease worsening with TAFINLAR + MEKINIST.*
As seen in 2 different studies, about half of patients taking each treatment were living without their disease worsening (known as progression-free survival) after:
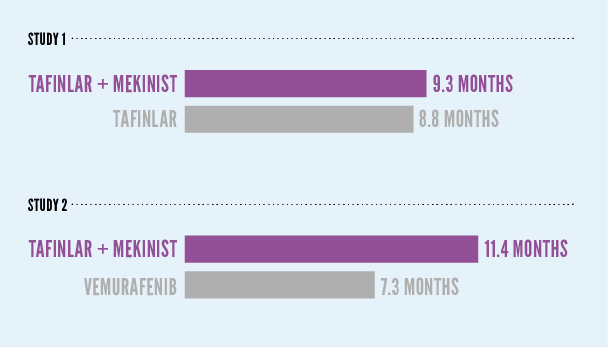
In Study 1, about half of patients taking TAFINLAR + MEKINIST were living without their disease worsening at 9.3 months, compared with 8.8 months for patients taking TAFINLAR alone. In Study 2, about half of patients taking TAFINLAR + MEKINIST were living without their disease worsening at 11.3 months, compared with 7.3 months for patients taking vemurafenib.
At 5 years, nearly a fifth of patients taking TAFINLAR + MEKINIST were living without their disease worsening†

Nearly 1 in 5 was living progression-free at 5 years.
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
More patients had their tumors shrink or disappear
In Study 1, more patients had their tumors shrink or disappear (known as the overall response rate) with TAFINLAR + MEKINIST.*
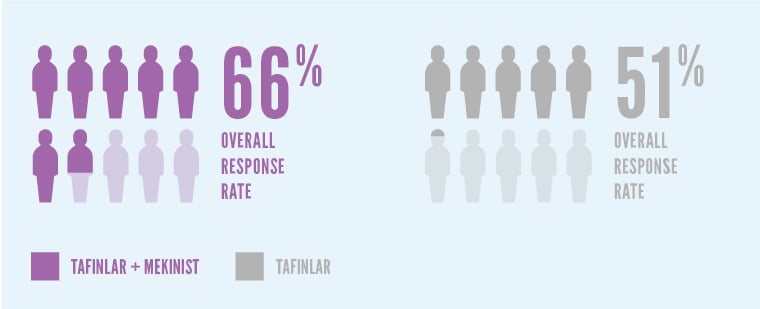
Patients taking TAFINLAR + MEKINIST saw a 66% overall response rate. Patients taking TAFINLAR alone saw a 51% overall response rate.
- Tumors disappeared completely in 10% of patients taking TAFINLAR + MEKINIST and 8% of patients taking TAFINLAR
- Tumors shrank in 56% of patients taking TAFINLAR + MEKINIST and 42% of patients taking TAFINLAR
- Tumors shrunk or disappeared for an average of 9.2 months with TAFINLAR + MEKINIST and 10.2 months with TAFINLAR
*As demonstrated by 2 studies: Study 1: a clinical trial that included 423 adult patients with BRAF V600E/K mutation-positive unresectable or metastatic melanoma. Patients were treated with a combination of TAFINLAR + MEKINIST or TAFINLAR alone. Study 2: a clinical trial that included 704 adult patients with BRAF V600E/K mutation-positive unresectable or metastatic melanoma. Patients were treated with a combination of TAFINLAR + MEKINIST or vemurafenib alone.
†As demonstrated in a pooled analysis of 2 clinical studies (Study 1 and Study 2) that included 563 adult patients with BRAF V600E/K-mutant unresectable or metastatic melanoma who were treated with a combination of TAFINLAR + MEKINIST.
TAFINLAR + MEKINIST is effective in advanced melanoma brain metastases
In a clinical study of people with advanced melanoma that had spread to their brain, half of people on TAFINLAR + MEKINIST saw their brain tumors shrink or disappear.‡
‡As demonstrated by a clinical trial that included 121 adult patients with BRAF V600E/K mutation-positive melanoma that had spread to the brain. Patients were treated with TAFINLAR + MEKINIST.
Helpful Terms To Know
To understand the importance of these results from clinical trials, it helps to know the terms and vocabulary. Learn the difference between overall survival, progression-free survival, and overall response rate.
OS describes the total time living with cancer after taking treatment. It means more time to live.
Progression-Free Survival (PFS)
PFS describes the amount of time the cancer doesn’t grow or spread while on treatment. It means living longer without the disease progressing.
ORR describes when tumors shrink or disappear as a result of treatment.


